Manager: Maria Luisa Minghetti
Heal Italia’s Biobanks and Sample Collections
HEAL Italia
A consortium of 72 partners dedicated to precision medicine in the fields of cancer, as well as cardiovascular, metabolic, and rare diseases.
Key Strength
A significant repository of human biological material, collected and preserved in research biobanks and sample collections.
Objective
To establish a network interconnecting the HEAL biobanks and promoting the use of biological materials according to the highest quality standards and ethical principles, in compliance with Italian legislation and international guidelines.
HEAL Italia Biobank Network
- CRO – National Cancer Institute of Aviano
- TLS Foundation
- IRCCS – University Hospital of Bologna
- IRCCS – National Cancer Institute Regina Elena
- IRCCS – Mario Negri Institute for Pharmacological Research
- Mediterranean Oncology Institute
- Italian National Institute of Health (ISS)
- Polytechnic University of Marche
- University of Catania
- University of Cagliari
- University of Foggia
- University of Milano-Bicocca
- University of Modena and Reggio Emilia
- Sapienza University of Rome – Dept. of Experimental Medicine
- University of Verona
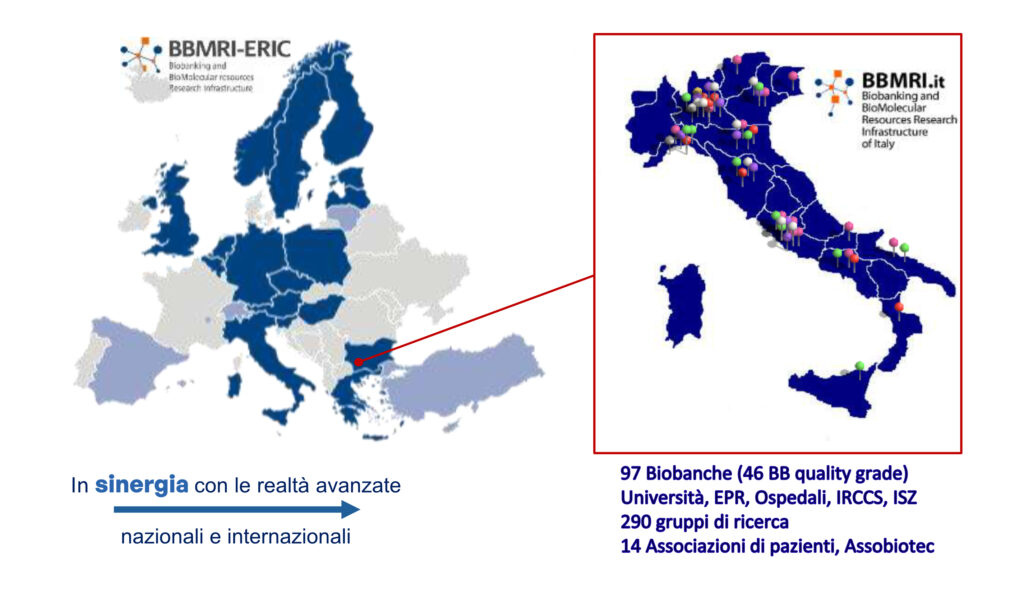
Strengthening of the Biobanking and Biomolecular Resources Research Infrastructure of Italy – CUP B53C22001820006
The PNRR project “Strengthening BBMRI.it” aims to reinforce the already extensive network of Italian biobanks within the BBMRI.it Research Infrastructure (www.bbmri.it), selected by the Ministry of University and Research (MUR) as a priority infrastructure under the National Research Infrastructure Plan (PNIR) 2021–2027.
Proper collection and quality preservation of human biological samples—and their associated data—are essential for conducting reproducible and effective translational research. To address this challenge, the European Union and Member States have funded a dedicated Research Infrastructure: BBMRI. Italy contributes through 97 biobanks, which are part of the national BBMRI.it node, 47 of which are recognized by the European hub, BBMRI-ERIC (bbmri-eric.eu).
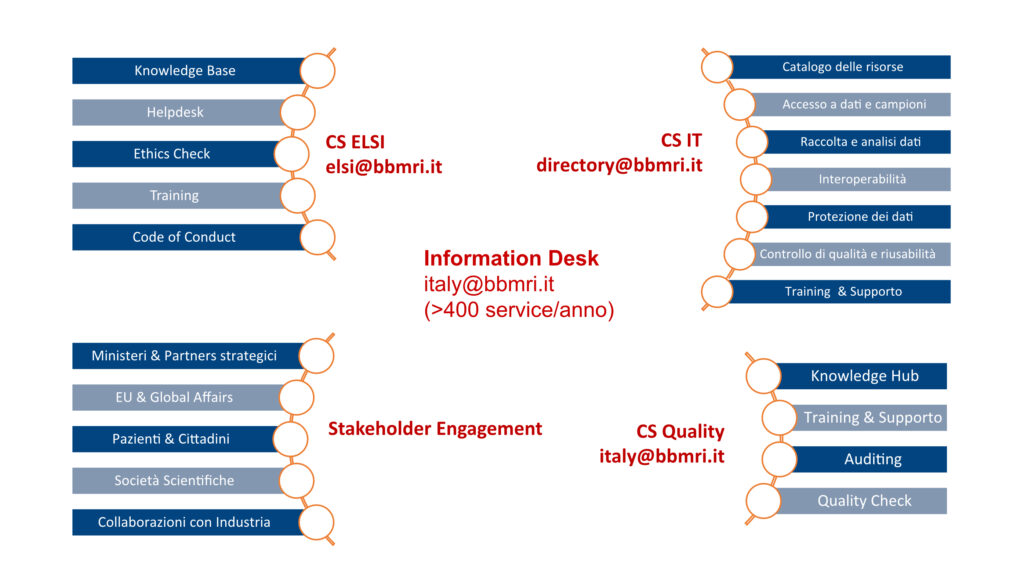
Distributed across the national territory, BBMRI.it offers access to biobank samples through a dedicated website, Help Desk, and specialized services in the areas of IT (Common Service IT), quality assurance (Common Service Quality), and ethical-legal-social issues (Common Service ELSI).
The “Strengthening BBMRI.it” PNRR project supports digital capacity building within biobanks, enhances the visibility of available biological resources in European and national catalogs, and fosters the development of new nodes within the infrastructure. Leveraging PNRR investments and the technical-scientific expertise of BBMRI.it, new biobanks will be established to specialize in collections such as organoids, microbiome, induced pluripotent stem cells (iPSC), and adult progenitor cells. The Italian Network of Core Facilities (NICo) will provide expertise to valorize biological samples made available by BBMRI.it through sample characterization activities.
The project also includes the strengthening of the Common Service ELSI and Common Service IT, and a dedicated Work Package on data standardization and FAIR principles, which will significantly enhance data accessibility and usability.
Furthermore, the project enables access to resources provided by the infrastructure through mechanisms aligned with Trans-National Access, the European model of Open Science that facilitates cross-border use of infrastructure resources.
Research Biobanks
Within a transparent and standardized framework, each Italian research biobank, through the BBMRI partner charter, presents its activities using a shared language, qualifies its operations, and publishes its ELSI tools (e.g., consent forms, Material Transfer Agreements), along with its governance practices (e.g., internal regulations, access policies, and return of results procedures).
Complex Operational Unit for Biobanks – BMS Biobank
The Biobank Operational Unit has been active at the Pisa University Hospital (AOUP) since 2016, with the aim of centralizing, maintaining, and enhancing internal biobanking activities. The Multispecialty Biobank (BMS) was formally established in March 2020 (AOUP-DelDG No. 272 of 27/03/2020) through an approved Organizational Document (DOA).
The BMS Biobank integrates the mission of the Departmental Biobanks Section, including both a clinical function (Tissue Institute and Regional Biological Archive) and a research function, serving as a transversal platform for AOUP departments and other scientific or healthcare institutions.
The structure is part of the BBMRI research infrastructure, certified ISO 9001:2015 and accredited ISO 20387 for the following human biological materials: serum, plasma, saliva, and nasopharyngeal tissue. BMS processes and stores each sample in accordance with national and international guidelines, ensuring traceability and adherence to quality standards, and linking clinical and scientific data.
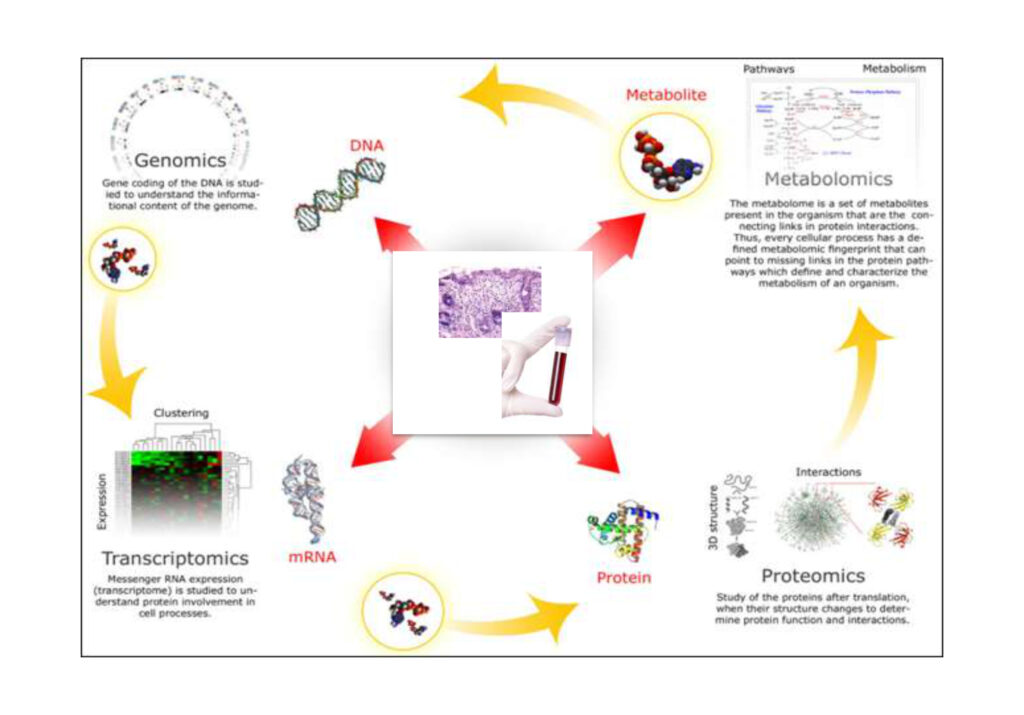
Human Biological Material, Omics Sciences, and Precision Medicine
The scientific information derived from human biological material, enabled by advancements in omics technologies, is central to the progress of translational research and precision medicine.
Much of today’s understanding of diseases, diagnostics, and therapies is based on studies using human biological material—defined as biological samples along with associated epidemiological, clinical, and experimental data.
High-quality human biological material is the most critical resource to translate molecular medicine and omics technologies into improved human health. The value of such material increases when combined with high-quality and comprehensive data.
Research Using Human Biological Material and Biobanks
Research involving human biological material must adhere to foundational ethical principles and be conducted transparently and with full respect for the individuals from whom the material originates.
Whenever possible, residual biological material should be preferred over new sample collection, always in compliance with ethical standards, participants’ rights, and minimum quality requirements.
Optimization of biological sample usage includes:
Use of best available techniques for preparation and storage
Integration of high-quality clinical data
Obtaining explicit informed consent for research purposes
It is the duty of the scientific community to foster trust among research participants and the wider public.
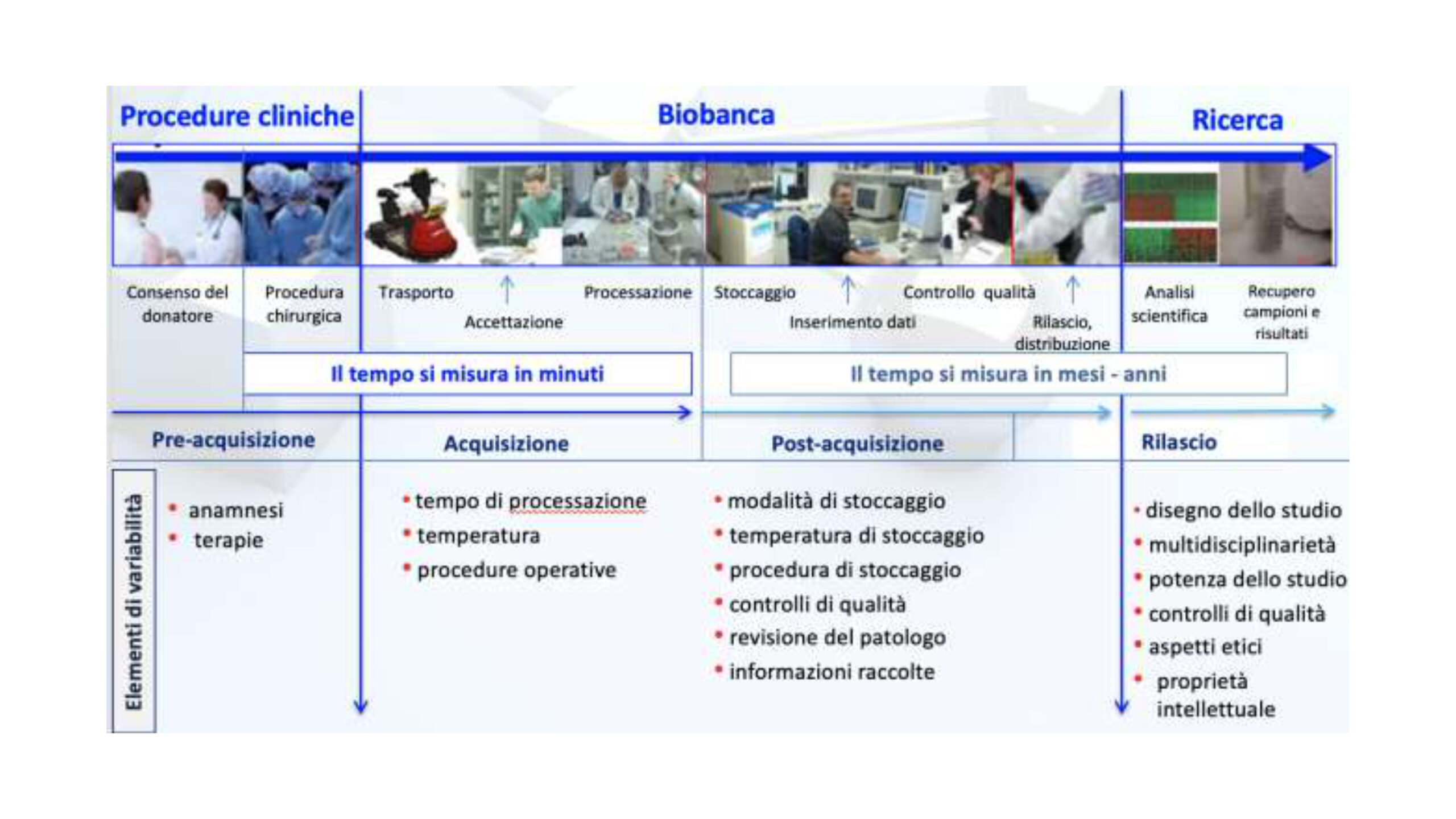
Research Biobanks: The Interface Between Patients and Medical Research
A research biobank plays a key role in ensuring both the quality of research and respect for ethical standards and participants’ rights.
Biobanking processes guarantee that biological material is collected and used in accordance with ethical and legal principles, and that sample quality is maintained through standardized procedures (SOPs). Sharing of samples and research findings (via access policies) is a cornerstone of biobank operations.
Research Biobank – Core Elements
- Respect for participant will: informed consent
- Data privacy protection
- Custody of samples, ensuring impartiality in sharing
- Transparent access criteria and project evaluation workflows
- Rules for transfer of samples/data and return of results
- Feedback and reporting on research outcomes
- Interoperability and integration in national/international networks
- Public communication and engagement with communities and patient organizations
Biobanks for Rare Disease Research
Rare diseases pose unique challenges due to the limited availability of samples and their diverse nature. Advancing research in this field depends on the preservation and transparent sharing of high-quality samples, with active involvement of rare disease communities in promoting and raising awareness of research biobanking.
Telethon Network of Genetic Biobanks (TNGB)
- 11 biobanks across Italy
- Founded in 2007 under a Telethon Foundation research project
- Over 120,000 biological samples covering ~1,000 rare genetic diseases
- Plays a central role at national and European levels:
– BBMRI-ERIC and the Italian Node
– EuroBioBank
– RD-Connect (FP7 2012–2018)
